Uranium Mitigation From Drinking Water
Uranium is a common constituent of many rocks in the Piedmont and mountains of NC. Recent well water testing in Eastern Wake County by the Wake County Department of Environmental Services has shown that elevated levels of uranium are present in many wells. This issue also extends to large portions of Franklin Count as well. This is due to the large bodies of granite that underlie these areas, which have elevated levels of uranium, radium and radon. Uranium is the heaviest naturally-occurring element on the Periodic Table with atomic number 92. All uranium is radioactive and the dominant uranium isotope is Uranium-238 (238U). 238U comprises about 99.27% of all naturally-occurring uranium. The other main isotope, comprising about 0.72% is 235U. Uranium was formed during the time when planets were first forming and since the uranium isotopes have unusually long half-lives, much of this material is still present on Earth. The half-life of 235U is 4.5 billion years and for 238U it is 700 million years. The reason the 238U is the most abundant over 235U is because of this much longer half-life ‑ this means it takes much longer to decay and disappear.
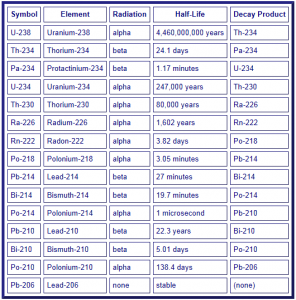
The radioactive decay path of 238U is shown in the diagram below. You can see a long progression of intermediate elements, or daughter products (progeny), that occur during the radioactive decay of uranium. From this table you can see that many of the daughter products themselves have very short half-lives. For example, 234Th decays in 24.1 days and 234Pa decays in 1.17 minutes. As these elements decay, radiation is given off, normally in the form of alpha particles, beta particles (see column 3 below) and gamma rays. These radioactive emissions are explained below.
Radioactive Emissions
Alpha particles
Alpha particles are essentially helium nuclei and are comprised of 2 protons and 2 neutrons. These particles are the least penetrating radiation given off during radioactive decay and are not dangerous in terms of external human exposure. That is, they can be stopped by a sheet of paper and are not energetic enough to penetrate human skin. However, they should not be consumed because once inside the body these particles can easily enter and damage cells. Therefore, the EPA has set a Maximum Contaminant Limit (MCL) limit for Gross Alpha at 15 picoCuries per liter (pCi/L).
Beta Particles
Beta particle are high-energy electrons that are emitted during radioactive decay and are more energetic than alpha particles. These can penetrate skin and cause cell damage and therefore contribute to radiation exposure. The EPA has set an MCL for beta particles are 4 millirems per year (mrem/yr). On the other hand, if inside the body, beta particles are not as dangerous because they are less likely to be absorbed by the cells and generally pass right through them.
Gamma Rays
Gamma rays are a high-energy form of electromagnetic radiation similar to X-rays. Gamma radiation is most dangerous when outside the body because it can penetrate skin and damage living cells. Gamma rays are another form of emission usually accompanying the main forms of radioactive decay.
How to Remove Uranium From Drinking Water
Based on the table above you can see that the decay of 238U involves many steps. The end result, if 238U is allowed to decay all the way, it eventually becomes 206Pb, which is normal stable lead. In terms of Drinking Water Standards, the most imports things that are regulated by the EPA are: Uranium, Radium, and Radon. Mitigation of radon and radium is discussed separately.
For uranium, the subject of this memo, the MCL set by the EPA is 30 mg/L (micrograms per liter). Since uranium is an alpha particle generator, removing it also reduces Gross Alpha. In drinking water uranium exists as complex anionic groups associated with carbonate:
- UO2(CO3)22−
- UO2(CO3)34−
- UO2(CO3)(OH)3−
You can also find a few commonly used ways for filtering Uranium from water through specialty water systems below.
Removal by Ion Exchange Water Softeners
These complex anions all have a negative charge associated with them and therefore can be removed using an ion-exchange process. For a point-of-entry system, a special strong base anion resin called A300E is used which can capture the uranium complexes. This system works just like a regular water softener except instead of removing calcium hardness, the anion exchange method removes uranium ions and usually achieves better than 98% reduction. It uses normal NaCl salt as the regenerant and the exchange is between the U-complexes and chloride ion (Cl–). For good uranium reduction it is important to have enough resin in the tanks for adequate contact time for the exchange. The uranium mitigation systems can be configured in one of two ways: 1) as a stand-alone system and 2) as a mixed resin bed system with A300E resin layered with normal water softener resin (C100E) for removal of hardness and radium.
The choice between using a mixed resin bed depends on the actual uranium concentration and also the level of hardness. If the hardness is high ‑ greater than 6-7 grains per gallon, using separate systems for uranium reduction and water softening is recommended to avoid mineralization inside the water softener valve.
If the hardness is low, then a mixed resin bed system works to solve both uranium and hardness (and radium) in a single system. This reduces the amount of salt that is used and also takes up a smaller footprint. The maintenance on a uranium ion exchange system is just like a water softener ‑ you must add salt periodically to regenerate the resin. The A300E resin should last up to 9 years if maintained properly and it is always a good idea to have an annual uranium test done to verify the levels in the treated water.
Removal by Reverse Osmosis
Since uranium is a heavy metal and is not absorbed by the skin, many people choose to remove the element only from their drinking water using a reverse osmosis drinking water system. Reverse Osmosis (RO) is the most common type of drinking water purification method to purify drinking and cooking water. These systems are generally point-of-use (POU) only and treat water at a designated faucet for drinking and cooking water only.
RO utilizes a semi-permeable membrane and effectively removes about 95% of ALL contaminants in drinking water. Since uranium is a heavy metal and large in size, it is easily remove by the RO process. RO also removes a huge number of other contaminants including all metals, heavy metals, radioactive metals, asbestos, silica, pharmaceuticals and other pollutants.
It is also possible to install a large RO system to treat all the water in the home (whole-house RO). This system is considerably larger and more expensive than the POU system and involves much larger membranes for better production rates, as well as large storage tanks and a repressurization system.
All RO systems have a discharge stream that carries away the contaminants rejected by the membrane and this stream is normally sent to the drain.
Test your water to determine if water treatment is necessary and which option is right for you.
